Crystal Structure of Aquaporins
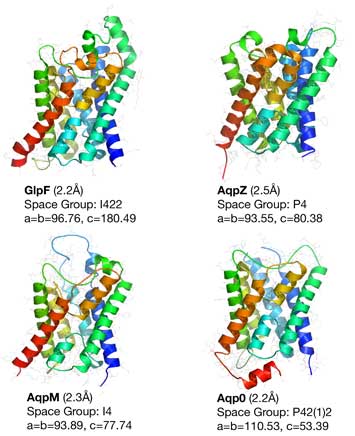
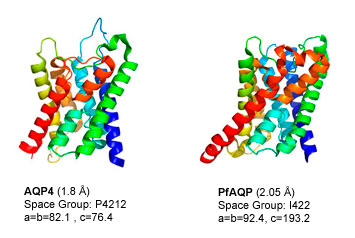
The six different aquaporin structures determined in the Stroud laboratory (E. coli GlpF, E. coli AQPZ, Bovine AQP0, Methanothermobacter marburgensis AQPM, Human AQP4, Plasmodium falciparum PfAQP are illustrated above. Crystallization is a gold standard for ability to clone, express, and adequately purify membrane proteins in a structurally homogeneous form. This therefore establishes that purification of these membrane proteins in structurally homogeneous form has been achieved by procedures used in the Stroud group.
Three of these aquaporins, Aqp0, AQP4, and PfAQP are eukaryotic, proving that there is nothing especially difficult about obtaining well behaved expression of higher animal membrane proteins. Human AQP4 was one of the first heterologously expressed human membrane proteins (expressed in Pichia pastoris yeast) The relatively high homology of the 11 human aquaporins (20-30% identity) (21) to their bacterial orthologs AqpZ , GlpF, and AQPM whose structure we solved suggests that we may be able to express other human eukaryotic proteins in yeast or bacteria expression systems.
Crystal Structure of Ammonia Channel
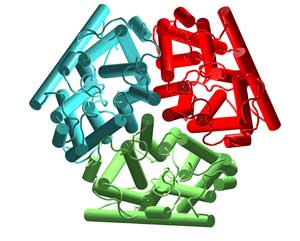
The top view of the monomeric AmtB is shown above. Each channel monomer is shown in a different color. The atomic structure of AmtB is the highest resolution of any membrane protein as we enter the year 2005. This is due to the procedures in use at the MPEC.
